Junshi Biosciences was established in Shanghai, the PRC.
Established in 2012, Junshi Biosciences is an innovation-driven biopharmaceutical company committed to developing first-in-class and best-in-class drugs through original innovation.
With an outstanding capacity for innovative drug discovery, advanced biotechnology research and development, a whole-industry-chain approach with large-scale production technology, and drug combinations with great market potential, Junshi Biosciences has endless potential in onco-immunotherapy as well as the treatment of autoimmune diseases, metabolic diseases, neurological diseases and infectious diseases.
Our Mission
With strong R&D capacity, Junshi Biosciences is at the cutting-edge of medical innovation. Our mission is toProvide patients with world-class, trustworthy, affordable, and innovative drugs.
-
To provide patients with treatment options that work better and cost less
-
To develop first-in-class and best-in-class drugs through original innovation
-
To become a pioneer in the area of translational medicine
-
To fulfill unmet medical needs and provide the best treatments for patients
Our Growth Strategy
-
Rapidly expand our
product pipeline -
Focus on the advancement and commercialization of
existing drug candidates -
Scale up our macromolecule fermentation capacity and
lower production cost
We aim to become an innovative and globally competitive biopharmaceutical company with a whole-industry-chain layout that encompasses R&D, manufacturing and commercialization.
Our Development Story
Year
- 2025
- 2024
- 2023
- 2022
- 2021
- 2020
- 2019
- 2018
- 2017
- 2016
- 2015
- 2014
- 2012
Our Leadership Team
Globalization
In China, For Global
Starting from its Shanghai Headquarters, Junshi Biosciences has established four Innovation Centers in China (Shanghai & Suzhou) and the US (San Francisco & Maryland), as well as two Production Bases in China (Suzhou & Shanghai). Junshi Biosciences has about 2,500 employees worldwide.
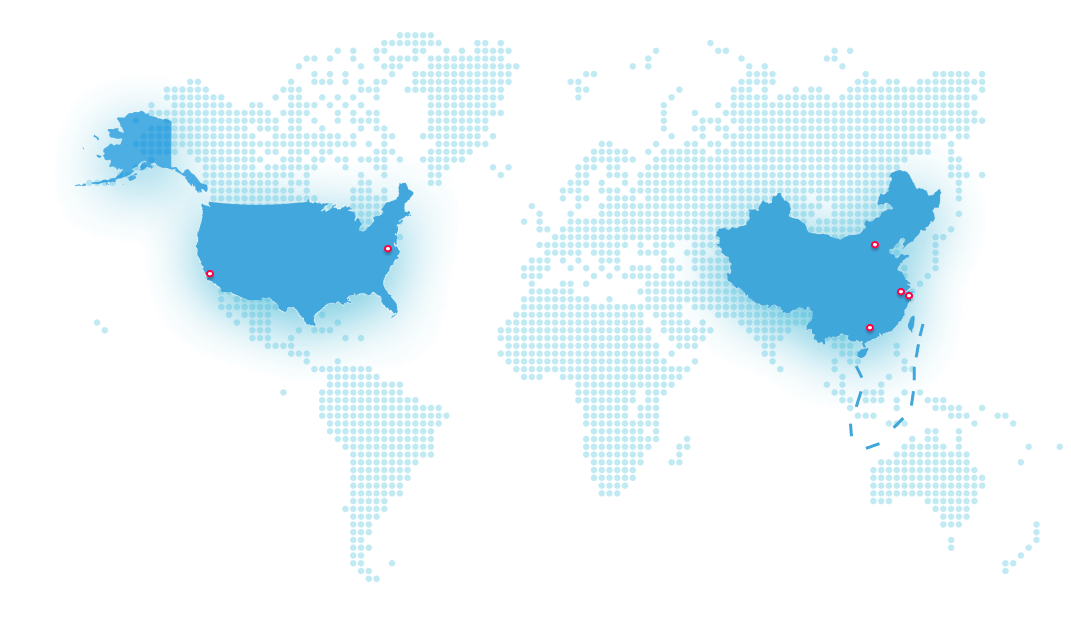
Headquarters
Shanghai Innovation Center
- Drug-target identification and verification
- Candidate molecule screening and optimization
- CMC process and analytical method development
- Technology transfer
- Drug registration and clinical development
Shanghai Lingang Production Base
- Establishment of stable cell line
- Process optimization
- cGMP standard
- Establishment and maintenance of global quality system
- Investigational products and commercial manufacturing
Suzhou Innovation Center
- Functional validation and process development of pipeline projects
- Pilot drug production
Suzhou Wujiang Production Base
- Establishment of stable cell line
- Process optimization
- GMP standard production
- Establishment and maintenance of global quality system
- Investigational products and commercial manufacturing
Maryland Innovation Center
- Novel drug target screening
- Functional antibody screening
- Analytical method development
- Technology transfer
- Drug registration and clinical development
San Francisco Innovation Center
- Novel drug target screening
- Candidate molecule screening
- Analytical method development
- Clinical biomarker research
- “Bioinformatics + AI” novel drug R&D
- Multi-relational data mining