Focusing on innovation and patient accessibility, we have established a diversified and complementary R&D pipeline. Most products are independently developed through our own platform, and we have a number of potential novel antitumor targets drugs. Our products respond to a vast array of diseases, including cancer, autoimmune, metabolic, neurological, and infectious diseases.
As we continue to enrich our pipeline and explore combination treatments, our fields of innovation R&D has expanded from monoclonal antibodies to small molecule drugs, polypeptide drugs, antibody-drug conjugates (ADCs), bispecific antibody or multispecific antibody drugs, nucleotide drugs and etc. We will continue to further explore the next generation of innovative therapies for cancer and autoimmune diseases.
R&D Pipeline
- Oncology
- Autoimmune
- Metabolic
- Neurological
- Infectious
- Global Developed
-
JS212
EGFR×HER3 ADC
Tumors
A recombinant humanized EGFR and HER3 bispecific ADC that is mainly used for the treatment of advanced malignant solid tumor
-
JS213
PD-1×IL-2
Tumors
A PD-1 and IL-2 bifunctional antibody fusion protein, which is mainly used for the treatment of advanced malignant tumors
-
JS207
PD-1+VEGF
Tumors
A recombinant humanized anti-PD-1/VEGF bispecific antibody self-developed by the company
-
JS203
CD3+CD20
Tumors
-
JS125
HDACs
Tumors
-
JS112
Aurora A
SCLC
-
JS111
EGFR exon 20
NSCLC
-
JS214
VEGF×TGF-β
Tumors
-
JS110
XPO1
Endometrial cancer
A small molecule inhibitor of the nuclear export protein XPO1
-
JS107
Claudin18.2 ADC
Gastrointestinal cancer
An antibody-drug conjugate (ADCs) targeting Claudin18.2 developed by Junshi Biosciences
-
JS105
PI3K-α
Gynecological tumors
An oral small molecule inhibitor targeting PI3K-α jointly developed by Junshi Biosciences and Risen Biosciences
-
JS015
DKK1
Tumors
A recombinant humanized anti-DKK1 monoclonal antibody developed independently by the company
-
JS009(TAB009)
CD112R/PVRIG
Tumors
A recombinant humanized monoclonal antibody against human CD112R developed independently by Junshi Biosciences
-
JS007
CTLA-4
Lung cancer, melanoma
A recombinant humanized anti-CTLA-4 monoclonal antibody developed independently by Junshi Biosciences
-
JS006(TAB006)
TIGIT
Tumors
A recombinant humanized anti-TIGIT monoclonal antibody developed independently by Junshi Biosciences
-
UBP1213sc
BLyS
Systemic lupus erythematosus
-
JT002
Small nucleic acid immunomodulator
Seasonal allergic rhinitis
A small nucleic acid immunomodulator jointly developed by Junshi Biosciences and its partner JSIAMA Biopharmaceutical
-
JS010
CGRP
Migraine
-
Tifcemalimab
BTLA
Lung cancer, lymphoma, etc
World’s first-in-human anti-BTLA mAb against tumor
-
JS005
IL-17A
Psoriatic, spondylitis
An anti-IL-17A monoclonal antibody developed independently by Junshi Biosciences
-
JS001sc
PD-1
Tumors
A subcutaneous injection formulation developed by Junshi Biosciences on the basis of toripalimab
-
Toripalimab
PD-1
Tumors
-
Adalimumab
TNF-α
Rheumatoid Arthritis, etc
-
Mindeudesivir Hydrobromide
RdRp
COVID-19
VV116 is an oral nucleoside analog drug that can inhibit the replication of SARS-CoV-2
-
Ongericimab
PCSK9
Hyperlipidemia
Ongericimab is a recombinant humanized anti-PCSK9 monoclonal antibody independently developed by Junshi Biosciences for the treatment of primary hypercholesterolemia and mixed dyslipidemia
Product Details
- Product name : TUOYI®
- Drug Code : Toripalimab
- Target : PD-1
- Equity : In-house
Toripalimab is an anti-PD-1 monoclonal antibody developed for its ability to block PD-1 interactions with its ligands, PD-L1 and PD-L2, and for enhanced receptor internalization (endocytosis function). Blocking PD-1 interactions with PD-L1 and PD-L2 promotes the immune system’s ability to attack and kill tumor cells.
More than forty company-sponsored toripalimab clinical studies covering more than fifteen indications have been conducted globally by Junshi Biosciences, including in China, the United States, Southeast Asia, and Europe. Ongoing or completed pivotal clinical trials evaluating the safety and efficacy of toripalimab cover a broad range of tumor types, including cancers of the lung, nasopharynx, esophagus, stomach, bladder, breast, liver, kidney, and skin.
In the Chinese mainland, toripalimab was the first domestic anti-PD-1 monoclonal antibody approved for marketing (approved in China as TUOYI®). Currently, there are ten approved indications for toripalimab in the Chinese mainland:
- unresectable or metastatic melanoma after failure of standard systemic therapy;
- recurrent or metastatic nasopharyngeal carcinoma (“NPC”) after failure of at least two lines of prior systemic therapy;
- locally advanced or metastatic urothelial carcinoma that failed platinum-containing chemotherapy or progressed within 12 months of neoadjuvant or adjuvant platinum-containing chemotherapy;
- in combination with cisplatin and gemcitabine as the first-line treatment for patients with locally recurrent or metastatic NPC;
- in combination with paclitaxel and cisplatin in first-line treatment of patients with unresectable locally advanced/recurrent or distant metastatic esophageal squamous cell carcinoma (“ESCC”);
- in combination with pemetrexed and platinum as the first-line treatment in EGFR mutation-negative and ALK mutation-negative, unresectable, locally advanced or metastatic non-squamous non-small cell lung cancer (“NSCLC”);
- in combination with chemotherapy as perioperative treatment and subsequently with monotherapy as adjuvant therapy for the treatment of adult patients with resectable stage IIIA-IIIB NSCLC;
- in combination with axitinib for the first-line treatment of patients with medium to high risk unresectable or metastatic renal cell carcinoma (RCC);
- in combination with etoposide plus platinum for the first-line treatment of extensive-stage small cell lung cancer (ES-SCLC);
- in combination with paclitaxel for injection (albumin-bound) for the first-line treatment of recurrent or metastatic triple-negative breast cancer (TNBC).
The ten indications have been included in the National Reimbursement Drug List (NRDL) (2024 Edition). Toripalimab is the only anti-PD-1 monoclonal antibody included in the NRDL for the treatment of melanoma, perioperative treatment of NSCLC, treatment of RCC and treatment of TNBC. In October 2024, toripalimab for the treatment of recurrent or metastatic NPC was approved in Hong Kong SAR, China.
Internationally, toripalimab has been approved for marketing in the United States, the European Union, India, the UK, Jordan, Australia and other countries and regions. In addition, toripalimab BLAs are under reviews in many countries around the global, including the Singapore Health Sciences Authority (HSA).
Mechanism of Action
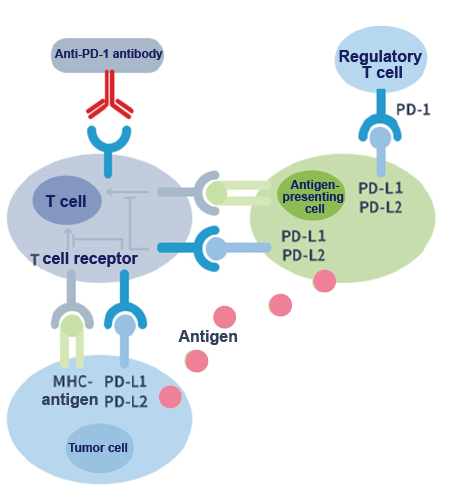
PD-1 is a T cell surface receptor; it is an immune checkpoint molecule in the co-inhibitory signal pathway of the T cell. As shown in the following figure, the binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to the inhibition of T-cell activation and immune surveillance of tumor cells. Anti-PD-1 monoclonal antibodies can block the PD-1/PD-L1 pathway and thereby restore the immune function of T cells.
Main research literature
- Serial number Journal name Document title Published on Link
- 1 JAMA Oncology Toripalimab For Extensive-Stage Small Cell Lung Cancer 2024 Open link
- 2 Journal of the American Medical Association Perioperative Toripalimab Plus Chemotherapy for Patients With Resectable Non-Small Cell Lung Cancer: The Neotorch Randomized Clinical Trial 2024 Open link
- 3 Nature Medicine Toripalimab plus nab-paclitaxel in metastatic or recurrent triple-negative breast cancer: a randomized phase 3 trial 2024 Open link
- 4 Journal of the American Medical Association Toripalimab Plus Chemotherapy for Recurrent or Metastatic Nasopharyngeal Carcinoma: The JUPITER-02 Randomized Clinical Trial 2023 Open link
- 5 Annals of Oncology Toripalimab plus axitinib versus sunitinib as first-line treatment for advanced renal cell carcinoma: RENOTORCH, a randomized, open-label, phase III study 2023 Open link
- 6 Cancer Cell Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial 2022 Open link
- 7 Journal of Clinical Oncology Toripalimab Plus Chemotherapy for Patients With Treatment-Naive Advanced Non–Small-Cell Lung Cancer: A Multicenter Randomized Phase III Trial (CHOICE-01) 2022 Open link
- 8 Journal for ImmunoTherapy of Cancer Toripalimab plus axitinib in patients with metastatic mucosal melanoma: 3-year survival update and biomarker analysis 2022 Open link
- 9 Clinical Cancer Research Safety, Efficacy, and Biomarker Analysis of Toripalimab in Patients with Previously Treated Advanced Urothelial Carcinoma: Results from a Multicenter Phase II Trial POLARIS-03 2022 Open link
- 10 Annals of Oncology Toripalimab (anti-PD-1) versus high-dose interferon-α2b as adjuvant therapy in resected mucosal melanoma: a phase II randomized trial 2022 Open link
- 11 Nature Medicine Toripalimab or placebo plus chemotherapy as first-line treatment in advanced nasopharyngeal carcinoma: a multicenter randomized phase 3 trial 2021 Open link
- 12 Signal Transduction and Targeted Therapy Toripalimab plus chemotherapy as second-line treatment in previously EGFRTKIs treated patients with EGFR-mutant advanced NSCLC: a multi-center phase II trial 2021 Open link
- 13 Journal of Clinical Oncology Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02) 2021 Open link
- 14 JAMA Network Open Safety, Antitumor Activity, and Pharmacokinetics of Toripalimab, a Programmed Cell Death 1 Inhibitor, in Patients With Advanced Non–Small Cell Lung Cancer A Phase 1 Trial 2020 Open link
- 15 Cancer Communications A Phase I Study of Toripalimab, an anti-PD-1 Antibody, in Patients With Refractory Malignant Solid Tumors 2020 Open link
- 16 Clinical Cancer Research Safety, Efficacy and Biomarker Analysis of Toripalimab in previously treated advanced melanoma: results of the POLARIS-01 multicenter phase II trial 2020 Open link
- 17 European Journal of Cancer Safety and clinical efficacy of toripalimab, a PD-1 mAb, in patients with advanced or recurrent malignancies in a phase I study 2020 Open link
- 18 mABs Glycosylation-independent binding of monoclonal antibody toripalimab to FG loop of PD-1 for tumor immune checkpoint therapy 2019 Open link
- 19 Acta Pharmacologica Sinica Preclinical evaluation of the efficacy, pharmacokinetics and immunogenicity of JS-001 2017 Open link
- 20 Clinical Cancer Research Efficacy, safety and biomarkers of toripalimab in patients with recurrent or metastatic neuroendocrine neoplasms:a multiple-center phase Ib trial 2020 Open link
- 21 Journal of Clinical Oncology Axitinib in Combination With Toripalimab, a Humanized Immunoglobulin G4 Monoclonal Antibody Against Programmed Cell Death-1, in Patients With Metastatic Mucosal Melanoma: An Open-Label Phase IB Trial 2019 Open link
- 22 Annals of Translational Medicine JS001, an anti-PD-1 mAb for advanced triple negative breast cancer patients after multi-line systemic therapy in a phase I trial 2019 Open link
- 23 Annals of Oncology Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemorefractory gastric cancer treated with toripalimab, a PD1 antibody in phase Ib/II clinical trial NCT02915432 2019 Open link
- 24 Journal of Hematology & Oncology Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients 2019 Open link